 |
BioSyM Research
Thrust 2: Multifunctional Cytometry
|
|
|
|
|
Cell Cycle Synchronization of Mesenchymal Stem Cells |
Homing and Engraftment of Culture Expanded MSCs |
High Throughput Separation of Circulating Tumor Cells (CTC) |
Palm Sized Nuclear Magnetic Resonance Relaxometry System for Point-of-Care Diagnosis |
Microwell Array Technologies for High Content Single Cell Cytometry |
We develop novel functional cell assays and cell manipulation methodologies, largely relying on physical or biological / physiological characteristics of cells that provide advantages for more accurate and faster means to isolate and understand the function of rare cells within body fluids and tissues. Specific projects include development of microfluidic magnetic resonance measurements to study redox/metabolomic states of cells noninvasively; integrated electrochemical and deformability measurements of cells to elucidate complicated redox changes occurring in blood and other tissues; microfluidic and mechanical cell sorting platforms applicable to clinical challenges in rare-cell-sorting, including isolation of circulating tumor cells; and microfabricated in situ cloning assays that include localized sensors of cellular function at all culture stages for rare and mixed cell types.
Capabilities Developed:
- Microfluidics device design and its fabrication in PDMS: PDMS material is the work horse for fabricating microfluidic devices for most of the research work in BioSyM.
- Microfluidics cell sorter technologies: A host of microfluidics devices designed for applications to isolate malaria affected cells, mesenchymal stem cells and cancer cells (circulating tumour cells or CTCs).
- Miniaturized, portable magnetic resonance relaxometry (MRR) system for point-of-care medical diagnosis of disease states such as malaria, dengue, enzyme deficiency, inflammation, sepsis.
People
|
Research Highlights
|
|
 |
...........The new technology developed by the SMART Centre's Inter‐Disciplinary Group, BioSystems and Micromechanics (BioSyM) will drive Clearbridge BioMedics’ next generation ClearCell™ System for circulating tumor cells (CTCs) isolation and retrieval from blood. Approximately 12.7 million new cancer cases were diagnosed around the world in 2008 with a projected increase in the number of cases to 20 million by 2030. Separating and detecting rare circulating tumor cells (CTCs) in blood early, before the tumor has metastasized, can significantly increase the patients’ survival chance. With the unique ability to retrieve these extremely rare cells intact, the ClearCell™ system will revolutionize personalized cancer treatment and companion diagnostics. |
1. Microfluidic cell sorter platform technologies
We designed and developed a microfluidics-based technology exploiting the interplay between centrifugal forces and lift forces to size fractionate several different cell types. The technique employs an inertial mechanism and can be extended to multiple applications such as sorting of malaria infected cells, circulating tumor cells (CTC) and size fractionating mesenchymal stem cells (MSCs). With this inertial cell sorter, we have achieved separation of human MSC populations at high throughput, (15 million cells/hr) into different stages of the stem cell cycle that can correlate with drug treatment efficacy, without compromising the cell viability (~95%). We have also separated red blood cells (RBCs) from patient samples in endometrial cell sample collection, which was efficacious because the RBCs were essentially a “contaminating” small cell type that interfered with diagnostics on other cell types in the mixture. In addition, we have developed an automated microfabricated deformability cytometer that is able to mechanically characterize a small number of ring stage malaria infected RBCs in a large population of uninfected RBCs [H-J], as a function of how much mechanical deformation of the RBCs is achieved during fluid-induced passage through stationary posts along the flow stream. The schematics below show different device designs suiting various applications.
|
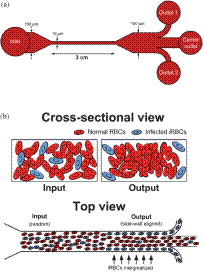
Microchannel design and separation principle developed for malaria infected RBCs (a) Schematic of the microfluidic design illustrating the device dimensions. Micro channels began with a 100 mm wide segment at the input that constricts to 15 mm. At the outlet, the microchannel opened into a 100 mm wide section for enhanced visualization with a three outlet bifurcation divided in 1 : 2 : 1 ratio. The microchannel height was fixed at 10 mm. (b) Schematic of the cross-sectional and top view of the microchannel illustrating the separation principle. The randomly distributed iRBCs (blue discoid cells) at the microchannel inlet marginate to the channel sidewalls as the flow reaches the outlet and are filtered out using a three outlet system. |
|
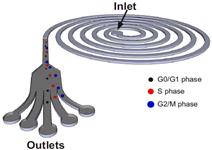
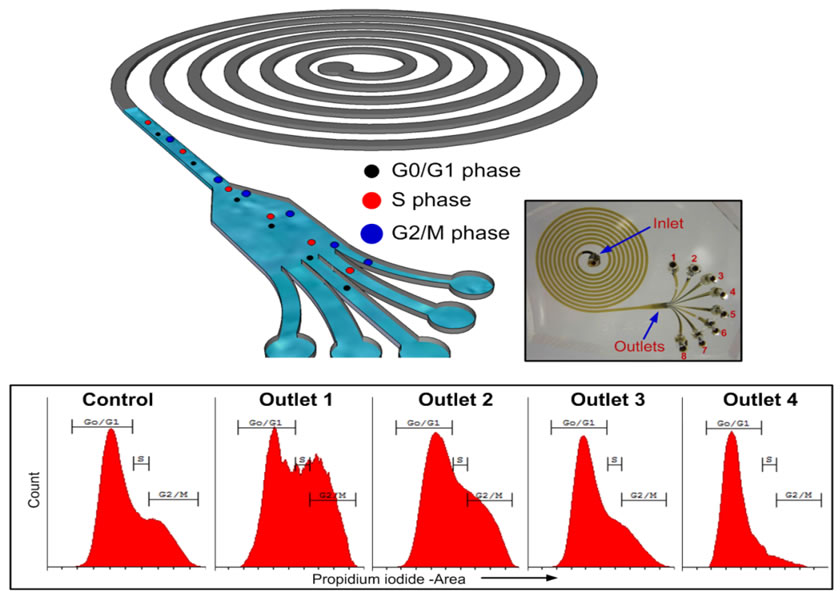
Schematic illustration of the spiral microfluidic design developed for cell cycle synchronization. Under the influence of inertial lift forces and Dean drag force, the hMSCs are size fractionated to obtain relatively pure populations of cells in the G0/G1, S and G2/M phase. The cells in the G2/M phase, due to the large size, equilibrate closest to the microchannel inner wall followed by cells in the S and G0/G1 phase. |
|
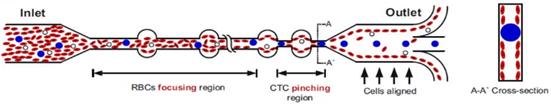
Schematic illustrating the erythrocyte focusing process in an high aspect ratio rectangular microchannel. The shear modulated inertial lift forces efficiently equilibrates all the cells along the channel side walls and are removed from the two side outlets. The channel design also consists of wider circular sections placed at regular intervals yielding higher channel structural integrity (preventing channel collapse due to high aspect ratio). By patterning a pinched region prior to the outlet, the larger CTCs are collected through the center outlet. |
|
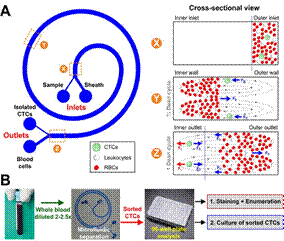
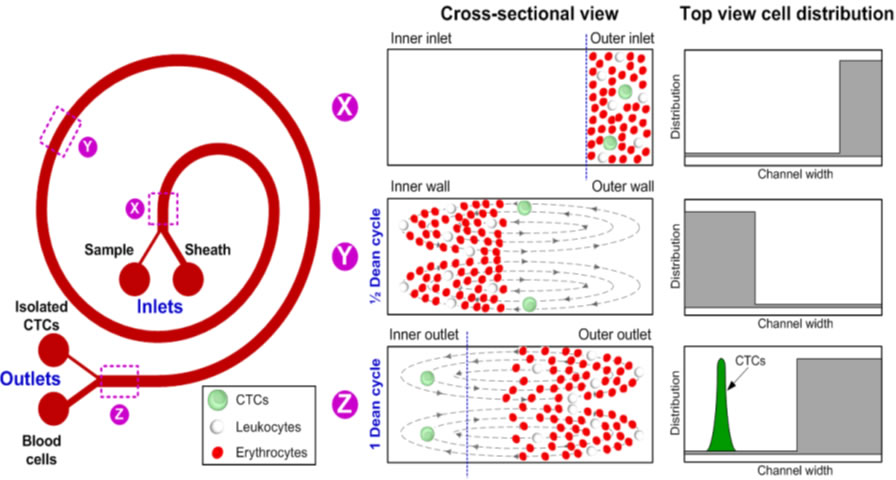
(A) Schematic illustration of the separation principle for high-throughput CTCs isolation using Dean Flow Fractionation (DFF). Blood sample and sheath fluid are pumped through the outer and inner inlets of the spiral device respectively. Under the influence of Dean drag forces (FD (blue arrows)), the smaller hematologic cells (RBCs and leukocytes) migrate along the Dean vortices towards the inner wall, then back to outer wall again (Dean cycle 1), while the larger CTCs experience additional strong inertial lift forces (FL (red arrows)) and focus along the microchannel inner wall, thus achieving separation. (B) Overall workflow of device operation and coupling with 96-well plate for various downstream applications such as CTCs enumeration and culture of sorted CTCs. Typical througput: 3 mL/hr |

|
Dual stage design for cell sorting at higher throughput rates. |
2. Spiral microchannel with trapezoidal cross-section for ultra-fast, label-free isolation of circulating tumor cells
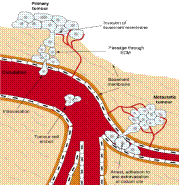
|
Circulating tumor cells (CTCs) are cancer cells detached from primary tumor & released into bloodstream through metastasis. |
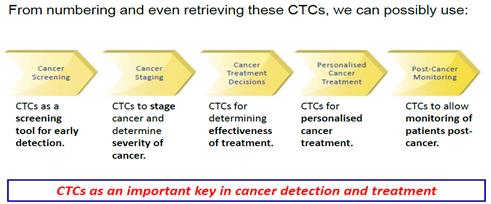
|
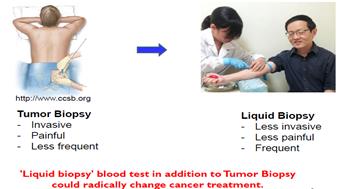
|
Our team developed a spiral microfluidic device for high throughput size-based separation and retrieval of CTCs from blood using the centrifugal forces coupled with inertial microfluidics phenomenon. This device, named as “Dean Flow Fractionation," has been successfully employed for isolation of CTCs from blood of patients with advanced metastatic non-small cell lung cancer (NSCLC). We have shown that our spiral biochip with rectangular cross-section is capable of processing blood with high hematocrit (~20-25%), thus achieving enrichment of rare cells at a processing speed of 3 mL/hr. Although, the throughput of our spiral biochip is much higher than existing microfluidic devices, in this work, we further improve the throughput of our spiral chip with a new designed spiral channel with trapezoidal cross-section. In contrast to the rectangular cross-section channels which require a sheath flow for particle dispersion inside the channels when the particle concentration is high, the trapezoidal channels requires only a single inlet for the sample and two outlets for waste and enriched cell collection during operation. Using this technique, we have successfully demonstrated enrichment of a high number of CTCs (3-125 CTCs/mL) from peripheral blood of patients with metastatic breast and lung cancer. This device can process 7.5 mL of red blood cell lysed blood in about 8-10 min, allowing enrichment of viable CTCs with relatively high purity and yield. The trapezoidal spiral channels can be produced with extremely low-cost and high resolution using conventional micro-milling and PDMS casting and can be operated using a single syringe pump, facilitating easy automation. We strongly believe that this novel strategy can be utilized for large-scale processing of clinical samples in order to enrich sufficient amount of CTCs for various detailed molecular analyses as well as clinical monitoring of individual patients undergoing therapy. The device is well suited to process even larger quantities of blood if required (20 mL in ~ 15 min), a growing need for obtaining large number of CTCs for multiple downstream tests. This work will be further continued in BioSyM-Phase 2 under Thrust 2 and the cross-thrust initiative on Anti-metastatic Cancer Treatments. Given the current scientific uncertainty of CTCs’ role in cancer pathophysiology, the flexibility enabled by our CTC enrichment technique would be critical in advancing the CTC-based cancer diagnostics.
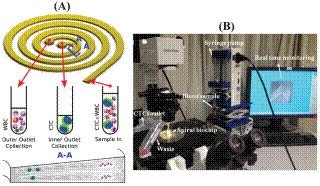
“Its rapid separation … is the highest throughput to date …and will allow large-scale processing of clinical samples.”
Chemistry Today, 2013.
|
(A) The operating principle of CTC enrichment by a spiral channel with a trapezoid cross-section (80 and 130 mm in the inner and outer channel height, respectively). CTCs focused near the inner wall due to the combination of the inertial lift force and the Dean drag force at the outlet while white blood cells (WBCs) and platelets are trapped inside the core of the Dean vortex formed closer to the outer wall. (B) The workstation setup for the CTC separation. The lysed blood is pumped through the spiral chip using a syringe pump where CTCs are separated from other blood components rapidly and efficiently. |
|
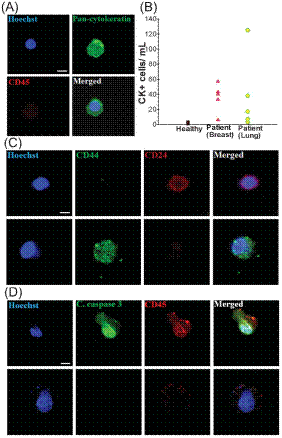
Blood from healthy donors (n = 5) as well as patients with metastatic breast and lung cancer (n = 10) was processed using the spiral chip. (A) Immunofluorescence staining of isolated CTC. CTC is identified by the following criteria: Hoechst positive, pan-cytokeratin positive and CD45 negative. (B) CTC enumeration plot for healthy donors (black), breast cancer patients (red), and lung cancer patients (green). (C) Identification of cancer stem cells (CSCs) in breast samples using standard markers. No CD44+/CD24+ cells were detected. It was found that CD44+ cells are larger than CD24+ cells. (D) Staining for apoptotic cells.Cleaved caspase-3 was absent in the isolated CTCs. The majority of the cells (.95%) expressing cleaved caspase-3 was CD45+. |
3. Microfluidics based sorting of mesenchymal stem cells (MSCs)
 |
Schematic illustration of the spiral microfluidic design developed for cell cycle synchronization. Under the influence of inertial lift forces and Dean drag force, the hMSCs are size fractionated to obtain relatively pure populations of cells in the G0/G1, S and G2/M phase. The cells in the G2/M phase, due to the large size, equilibrate closest to the microchannel inner wall followed by cells in the S and G0/G1 phase. |
|
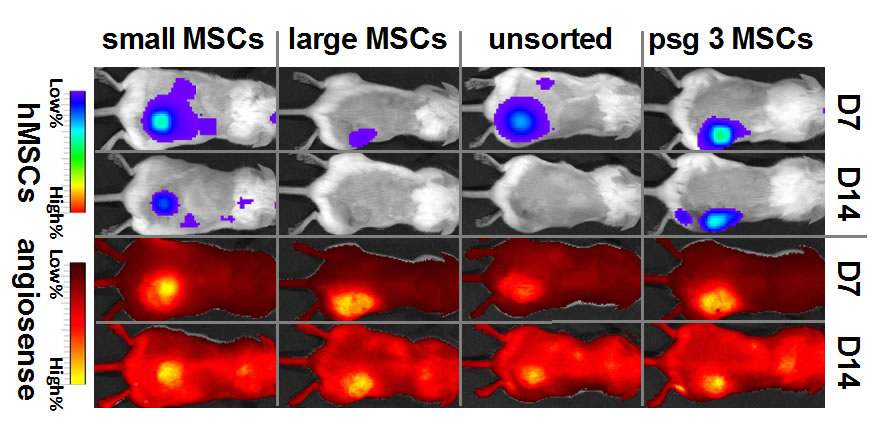
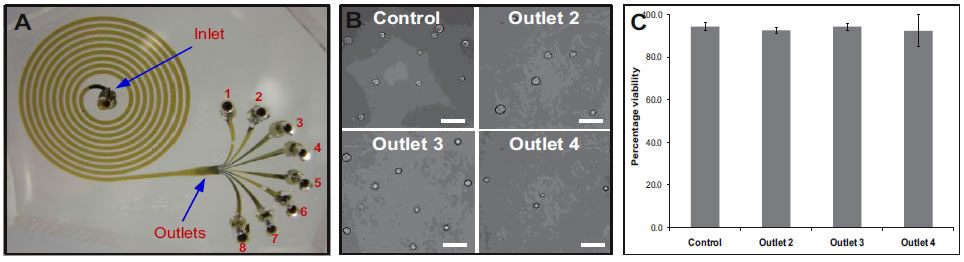
| A. Photograph of the spiral microchannel with one inlet and eight outlets fabricated in PDMS. B. Optical micrographs of the size sorted cells collected from outlets 2, 3 and 4. bar = 50μm. C. Viability of sorted hMSCs verified using trypan blue exclusion assay. Results indicate that the shear experienced at very high flow rates (2 mL/min), do not compromise the cells achieving >90% cell recovery. |
4. On-Chip Magnetic Resonance for single cell level detection
Magnetic Resonance (MR) Spectroscopy-Imaging is a mature and developed technology, well-known for its non-invasive analysis. Currently, conventional MR systems often require large sample volumes (in the order of mL) and bulky, expensive, high-field magnets, due to its low signal-to-noise ratio and low sensitivity which are however, not the inherent limitation of MR principle. With the advent of microfabrication and microfluidic technology, miniaturized magnetic resonance (MR) systems have been developed, with much higher sensitivity in terms of sample mass, and comparable sensitivity in terms of target concentration. This was achieved by miniaturizing the conventionally large detection coil to a micro-sized coil (often termed as microcoil) combined with microfluidic sample channels enjoying high filling factor. This project is an attempt to design, fabricate and develop a functional and portable medical diagnostic tool based on the principle of magnetic resonance.
5. Directional cell migration in an extracellular pH gradient
Extracellular pH (pHe) gradients are characteristic of tumor and wound environments. Cell migration in these environments is critical to tumor progression and wound healing. We have previously shown that cell migration can be modulated in conditions of spatially invariant acidic pHe due to acid-induced activation of cell surface integrin receptors, but the effects of pHe gradients on cell migration remain unknown. Here, we investigate cell migration in an extracellular pHe gradient, using both model αvβ3 CHO-B2 cells and primary bovine retinal microvascular endothelial cells. For both cell types, we find that the mean cell position shifts toward the acidic end of the gradient over time, and that cells preferentially polarize toward the acidic end of the gradient during migration. We adapted our previous computational cell migration model to include pHe-dependent integrin activation, and the model predicted that acid-induced integrin activation alone could produce directional migration down a pHe gradient. With experiments, we further demonstrate that cell membrane protrusion stability and actin-integrin adhesion complex formation are increased in acidic pHe, which could contribute to the preferential polarization toward acidic pHe that we observed for cells in pHe gradients. These results provide the first demonstration of preferential cell migration toward acid in a pHe gradient, with intriguing implications for directed cell migration in the tumor and wound healing environments.
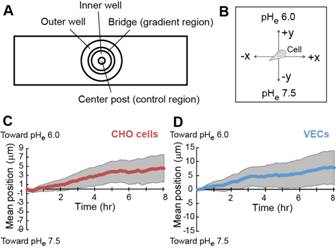 |
Migration of αvβ3 CHO-B2 cells in a pHe gradient. (A) Schematic top-down illustration of the Dunn chamber used to establish pHe gradients. When the inner and outer wells are filled with solutions of different concentrations, a linear H+ concentration gradient forms across the bridge. (B) Illustration of the coordinate system used for data analysis. For cells in the pHe gradient, the y-axis is parallel to the gradient direction and the x-axis is perpendicular to the gradient direction. (C & D) Mean y-coordinate (line) with 95% CI (gray shaded regions) over time for αvβ3 CHO-B2 cells (red) and VECs (blue) in the pHe gradient. The mean y-coordinate is significantly different than zero for all timepoints at which the error bars do not encompass the x-axis |
A novel, compact-sized (19 cm × 16 cm) and portable (500 g) magnetic resonance relaxometry system is designed and developed. We overcame several key engineering barriers so that magnetic resonance technology can be potentially used for disease diagnosis-monitoring in point-of-care settings, directly on biological cells and tissues. The whole system consists of a coin-sized permanent magnet (0.76 T), miniaturized radio-frequency microcoil probe, compact lumped-circuit duplexer, and single board 1-W power amplifier, in which a field programmable gate array -based spectrometer is used for pulse excitation, signal acquisition, and data processing. We show that by measuring the proton transverse relaxation rates from a large pool of natural abundance proton-nuclei presence in less than 1 μL of red blood cells,............................
7. Sphingolipid Biochemistry of Endometriosis Pathophysiology
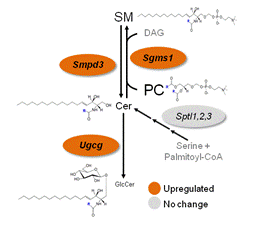
Endometriosis is a chronic gynecological disease of complex etiology, and has a complex interaction with infertility and significant socio-economical burden. However, the molecular mechanisms underlying this establishment and subsistence of ectopic lesions outside the endometrium remain enigmatic. We leveraged BioSyM’s expertise in mass spectrosmetry and the cell-based nature of this disease to understand the association of serum, peritoneal fluid and tissue sphingolipids in endometriosis pathophysiology. Mass spectrometry-based sphingolipidomics identified the accumulation of numerous sphingolipids. The upregulation of specific endometrial sphingolipid enzymes resulted in the dysregulated metabolism of these sphingolipids, which was associated with decreased endometrial apoptosis and reduced fecundity in endometriotic women. The functionality of glucosylceramides as a mitogenic factor has been determined. Potential translational implication of our discovery includes the development of novel direct therapeutics against endometriosis and non-invasive biomarkers of diagnostic value.
 A A
|
 B B
|
Cer, ceramide; GlcCer, glucosylceramide; PC, phosphatidylcholine; SM, sphingomyelin. SMPD3,sphingomyelinase 3, GCS, glucosylceramide synthase, SMS1, sphingomyelin synthase 1, SMPD1, sphingomyelinase 1 |
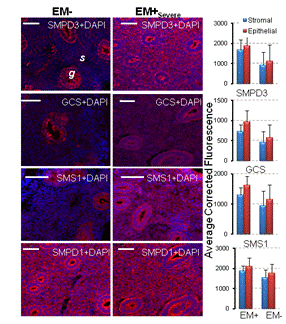
Regulation of sphingolipid concentrations in endometriosis in the endometrium. (A) Serum lipid concentrations and lipid enzymes that control their metabolism in the endometrium of severe endometriosis (EM+Sev) and endometriosis-free (EM-). Font size is proportionate to the abundance of the lipids. Unmeasured analytes are shown in grey. (B) Representative immunohistochemistry of endometrial sphingolipid enzymes in EM- and EM+Sev. Sections of endometrium were stained with SMPD3, GCS, SMS1 and SMPD1. White bar=50 µm, g=gland, s=stromal. |
8. CD26/DPPIV Downregulation in Endometrial Stromal Cell Migration in Endometriosis
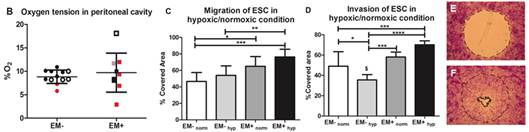
Endometriosis is characterized by the implantation of ectopic endometrial tissue outside the uterine cavity, usually onto pelvic peritoneum, ovaries or recto-vaginal septum. Although the pathophysiology is unknown, endometriosis is likely multifactorial involving interplay between genetic predisposition and environmental conditions leading to the pathogenesis. Endometrial stromal cells (ESCs) could regulate both normal and pathological endometrial behavior, engage in extensive crosstalk with the epithelia and respond to a convolution of hormonal and inflammatory cues. In endometriosis, stromal enlargements are found in endometriotic nodules and may serve as new sites for endometrial glandular growth. Oxygen tension likely varies throughout the menstrual cycle and is critical for regulating vasculogenic peptides such as vascular endothelial growth factor (VEGF).
On the basis of the findings stated and that previous ESCs migration studies were all done exclusively in normoxic culture conditions, we hypothesized that ESCs derived from the eutopic endometrium in patients with endometriosis are more migratory at physiological oxygen tensions present in the peritoneal cavity, in other words hypoxia. We found evidence that peritoneal cavity of women is generally hypoxic regardless of whether or not they have endometriosis. Under in vitro hypoxia, ESCs’ migratory behaviors from endometriosis patients differ significantly from those derived from healthy subjects. More detailed studies revealed the down-regulation of ectoenzyme CD26/DPPIV and up-regulation of angiogenic factors such as VEGF and CXCL6 by hypoxia in endometriotic ESCs. A more aggressive pattern of ESCs in cell migration and invasion assays verified the involvement of CD26-mediated pathways. These findings would serve as a platform for understanding the pathophysiology of endometriosis in terms of ESCs migration and invasion contributing to the lesion development.

|
|
Migration and Invasion under physiological oxygen tension in endometriosis. (A) Schematic flow chart of the study. (B) Oxygen tension in peritoneal cavity was evaluated by measuring peritoneal fluid %O2 in EM+ and EM- women. Fluid was collected at the beginning of the surgery and measured immediately by an automated blood gas analyzer. White: proliferative; grey: irregular; black: secretory, red: menses. Data were expressed as the mean SD. Migration (C) and invasion (D) of EM+ and EM- ESCs was examined under hypoxia (20% O2) or normoxia (20% O2). Cells were seeded in Oris Procell migration/ invasion assay and were allowed to attach under hypoxic or normoxic condition for 2 hours (E). For invasion, collagen gel was coated onto the well after cell attachment. Seeded cells after 48 hrs (F) were fixed and stained with crystal violet followed by measurement of the %covered area. Results were expressed as the mean ±SD. n=7, 7. *p<0.05 **p<0.005 ***p<0.001****p<0.0005; $p<0.05 as determined by paired t-test of same samples |
9. Fetal extracellular matrices for ex vivo regeneration of adult mesenchymal stem cells for scientific and clinical applications
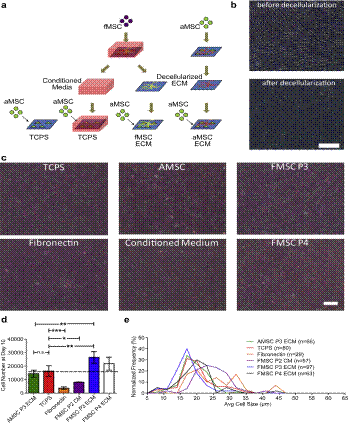
Expanding large-scale highly functional adult human mesenchymal stem cells (MSCs) has been challenging in regenerative medicine and gene therapy applications. This is because it is well recognized that the MSCs lose their ability to self-renew as well as their multipotency upon long-term culture on tissue culture plastic. There are recent studies that suggest that de-cellularized ECM can provide a microenvironment or niche for stem cell self-renewal capacity. The ECM used in these studies are from adult sources. Based on previous published observations from Dr. J. Chan's lab that fetal MSCs demonstrated superior proliferation and osteogenic potential in comparison to their adult counterparts, we hypothesized that (1) fetal MSC-derived ECM may improve the expansion of adult MSCs compared to traditional TCPS substrates and adult-derived ECM and (2) self renewal and multipotency of aged or older passaged adult MSCs can be improved by culturing on ECM derived from fetal MSCs. We demonstrated that fMSC ECM promotes aMSC expansion better than the other conditions including TCPS, aMSC ECM and nHDF ECMs in terms of superior growth, smaller and more uniformed-sized cells. This phenomenon may be dependent on the age (passage number) of the cells producing the substrate. Late-passage aMSCs (previously cultured on TCPS for 5 passages) were significantly improved on the fMSC ECM platform in terms of proliferation, stemness and differentiation potential compared to continuous culture in standard TCPS conditions. We also quantified amount of ECM produced by each plated cell, which suggested feasible scalability for clinical applications. The results established fMSC matrices as promising platforms for ex vivo expansion of human aMSCs.
|
(a) Schematic of decellularized ECM study. MSCs are plated on TCPS and cultured to generate extracellular matrices. The MSCs are decellularized to expose the layer of ECM for proliferation studies with aMSCs. In addition, media is collected from the fMSC cultures for the conditioned media (CM) condition. To assess proliferation, aMSCs of passage 4 were seeded on TCPS controls and decellularized matrices, cultured for 10 days before detachment to evaluate cell number and cell size. (b) Micrograph showing cell monolayer before and after the decellularization process. The decellularization protocol successfully remove the cells leaving a layer of extracellular matrices. Scale Bar ¼ 200 mm. (c) Micrographs showing adult mesenchymal stem cells cultured on the various conditions. Qualitatively, aMSCs cultured on decellularized matrices are morphologically similar to cells cultured on TCPS. Scale Bar ¼ 200 mm. (d) Cell yield (n ¼ 3) and (e) size distribution of aMSCs cultured under various conditions. Statistical analysis with 1-way ANOVA with Tukey’s Multiple Comparison Test; *p 0.05, **p 0.01, ***p 0.001. |
|
Journal Publications
|
|
Conference Presentations / Publications
|
- Constantine Tzouanas (Rice Univ., TX, USA), Joey Sze Yun Lim (SMART), Ya Wen (Plug & Play Tech Centre, CA, USA), J.P.Thiery (NUS), Bee Luan Khoo (SMART), "Microdevices for Non-Invasive Detection of Bladder Cancer", Chemosensors 2017, 5(4), 30
- Lykov, K. (NUS), Nematbakhsh Y. (NUS), Shang, M. (SMA3/NUS), Lim, C. T. (NUS), Pivkin, I. (NUS), “Probing eukaryotic cell mechanics via mesoscopic simulations.” PLOS Computational Biology 2017 Sep 18;13(9):e1005726. doi: 10.1371/journal.pcbi.1005726. [Epub ahead of print]
- Rou Jun Toh (NTU/SMART), Carmen C. Mayorga-Martinez NTU), Jongyoon Han (MIT/SMART), Zdenek Sofer, and Martin Pumera (NTU), "Group 6 Layered Transition-Metal Dichalcogenides in Lab-on-a-Chip Devices: 1T-Phase WS2 for Microfluidics Non-Enzymatic Detection of Hydrogen Peroxide", Analytical Chemistry, April 10 (2017) - Published online
- Rou Jun Toh (SMA3/NTU), Carmen C. Mayorga-Martinez (NTU), Zdenek Sofer (NTU), and Martin Pumera (NTU), “1T-Phase WS2 Protein-Based Biosensor”, Adv. Funct. Mater. 2017, 10.1002/adfm.201604923
- Rhokyun Kwak (KIST, Korea), Van Sang Pham (SMART), and Jongyoon Han (MIT/SMART), “Sheltering the perturbed vortical layer of electroconvection under shear flow,” Journal of Fluid Mechanics, 813, 799-823 (2017)
- Rou Jun Toh* (SMA3/NTU), Carmen C. Mayorga-Martinez (NTU), Zdenek Sofer (NTU), and Martin Pumera (NTU), “MoSe2 Nanolabels for Electrochemical Immunoassays, Analytical Chemistry, 88 (24), 12204 (2016).
- Bee Luan Khoo (SMART), Gianluca Grenci (MBI/NUS), Tengyang Jing (NUS/SMART), Ying Bena Lim (NUS), Soo Chin Lee (NUHS), Jean Paul Thiery (NUS), Jongyoon Han (MIT/SMART), Chwee Teck Lim (NUS/SMART), "Liquid biopsy and therapeutic response: Circulating tumor cell cultures for evaluation of anticancer treatment", Science Advances, 2 : e1600274 (2016)
- Naresh Kumar (NUH), Aye Sandar Zaw (NUH), Bee Luan Khoo (SMART), Sayantani Nandi (SMART), Zhangxing Lai (NUS), Gurpal Singh (NUH), Chwee Teck Lim (NUS/SMART), Jean Paul Thiery (NUS), "Intraoperative cell salvage in metastatic spine tumour surgery reduces potential for reinfusion of viable cancer cells", Eur. Spine J., Published online March 2016
- Bee Luan Khoo (MBI/SMART), Parthiv Kant Chaudhuri (MBI), Naveen Ramalingam (Fluidigm, CA), Daniel Shao Weng Tan (NCCS, A*STAR-GIS), Chwee Teck Lim (MBI/SMART/NUS) and Majid Ebrahimi Warkiani (SMART/UNSW), "Single-cell profiling approaches to probing tumor heterogeneity", International Journal of Cancer, Published online Jan 2016
- Andy Tay (NUS/SMART/UCLA), Andrea Pavesi (SMART), Saeed Rismani Yazdi (Univ.of Milan), Chwee Teck Lim (NUS/SMART), Majid Ebrahimi Warkiani (SMART/UNSW), "Advances in microfluidics in combating infectious diseases", Biotechnology Advances, Published Online (2016); http://dx.doi.org/10.1016/j.biotechadv.2016.02.002
- EMMA S. LUCAS, NIGEL P. DYER, KEISUKE MURAKAMI, YIE HOU LEE, YI-WAH CHAN, GIULIA GRIMALDI,JOANNE MUTER, PAUL J. BRIGHTON, JONATHAN D. MOORE, GNYANESHWARI PATEL, JERRY K.Y. CHAN, SATORU TAKEDA, ERIC W-F. LAM, SIOBHAN QUENBY, SASCHA OTT, JAN J. BROSENS,"Loss of Endometrial Plasticity in Recurrent Pregnancy Loss", Stem Cells, 34, 346–356 (2016)
- Majid Ebrahimi Warkiani (SMART/UNSW), Bee Luan Khoo (SMART), Lidan Wu (MIT), Andy Kah Ping Tay (NUS), Ali Asgar S Bhagat (Clearbridge), Jongyoon Han (MIT/SMART) & Chwee Teck Lim (NUS/SMART), "Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics", Nature Protocols, 11 (1), 134 (2016)
- C. P. Lim (NTU/SMART), J. Han (MIT/SMART) and Y. C. Lam (NTU), "Chaos analysis of viscoelastic chaotic flows of polymeric fluids in a micro-channel", AIP ADVANCES 5, 077150 (2015)
- Majid Ebrahimi Warkiani (SMART/Univ of NSW), Andy Kah Ping Tay (NUS), Guofeng Guan (SMART/Clearbridge), Jongyoon Han (MIT/SMART), "Membrane-less microfiltration using inertial microfluidics", Scientific Reports, 5, 11018 (2015)
- Tian Fook Kong (NTU/SMART), WeijianYe (NTU/SMART), Weng Kung Peng (SMART), Han Wei Hou (Medical School, NTU), Marcos (NTU), Peter Rainer Preiser (NTU), Nam-Trung Nguyen (Griffith Univ.) and Jongyoon Han (MIT/SMART), "Enhancing malaria diagnosis through microfluidic cell enrichment and magnetic resonance relaxometry detection", Scientific Reports (Nature Publishing), 5, 11425 (2015)
- Bee Luan Khoo (MBI), Soo Chin Lee (NUS/CSIS), Prashant Kumar (A*STAT IMCB), Tuan Zea Tan (CSIS), Majid Ebrahimi Warkiani (SMART), Samuel GW. Ow (NUS), Sayantani Nandi (A*STAR IMCB), Chwee Teck Lim (NUS/SSMART),
Jean Paul Thiery (NUS/A*STAR IMCB), "Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy", Oncotarget (Published Online, May 2015)
- Lidan Wu (MIT), Allison M. Claas (MIT), Aniruddh Sarkar (MIT), Douglas A. Lauffenburger (MIT) and Jongyoon Han (MIT/SMART), "High-throughput protease activity cytometry reveals dose-dependent heterogeneity in PMA-mediated ADAM17 activation", Integrative Biology, 7, 513 (2015)
- Majid E.W. (SMART), Andy Kah Ping Tay (NUS), Bee Luan Khoo (NUS/MBI), Xu Xiaofeng (NUS), Jongyoon Han (MIT/SMART), C.T.Lim (NUS/SMART), "Malaria Detection using Inertial Microfluidics", Lab on A Chip, 15, 1101-1109 (2015)
- Zhiyong Poon (SMART), Wong Cheng Lee (SMART), Guofeng Guan (NUS), Lin Myint Nyan (SMART), Chwee Teck Lim (NUS/SMART), Jongyoon Han (MIT/SMART) and Krystyn J. Van Vliet (MIT/SMART), “Bone Marrow Regeneration Promoted by Biophysically Sorted Osteoprogenitors From Mesenchymal Stromal Cells”, Stem Cells Translational Medicine, Published online on 19th Nov 2014, doi: 10.5966/sctm.2014-0154
- Tengyang Jing (NUS/SMART), Ramesh Ramji (NUS), Majid Ebrahimi Warkiani (SMART), Jongyoon Han (MIT/SMART), Chwee Teck Lim (NUS/SMART), Chia-Hung Chen (NUS), "Jetting microfluidics with size-sorting capability for single-cell protease detection", Biosensors and Bioelectronics, Published online, Nov 2014
- Wong Cheng Lee (NUS/SMART), Hui Shi (SMART), Lin Myint Nyan (SMART), Tanwi Kaushik (NUS/SMART), G.V.Shivashankar (NUS/MBI), Jerry Chan (Duke-NUS/KKH), C.T.Lim (NUS), Jongyoon Han (MIT/SMART), Krystyn Van Vliet (MIT/SMART), "Multivariate biophysical markers predictive of mesenchymal stromal cell multipotency", PNAS, Oct 2014, Published Online, doi: 10.1073/pnas.1402306111
- Weng Kung Peng (SMART), Tian Fook Kong (NTU/SMART), Chee Sheng Ng (NTU), Lan Chen (SMART), Yongxue Huang (SMART), Ali Asgar S Bhagat (SMART), Nam-Trung Nguyen (NTU), Peter Rainer Preiser (NTU) & Jongyoon Han (MIT/SMART), Micromagnetic resonance relaxometry for rapid label-free malaria diagnosis, Nature Medicine, Published online, 31 August 2014
- Rou Jun Toh (NTU/SMART), Weng Kung Peng (SMART), Jongyoon Han (MIT/SMART) & Martin Pumera (NTU), "Direct In Vivo Electrochemical Detection of Haemoglobin in Red Blood Cell", Scientific Reports (Nature Publishing), Vol. 4, Article number: 6209, 2014.
- Maloney, J.M. (SMART/MIT), and Van Vliet, K.J. (MIT/SMART), “Chemo-environmental modulators of fluidity in the suspended biological cell” Soft Matter, 10, 8031-8042, 2014.
- Khoo, B L (NUS/MBI), E W Majid (SMART), D S W Tan (NCCS), A A S Bhagat (Clearbridge), D Irwin (Sequenom Inc., CA), D P Lau (NCCS), A S T Lim (SGH), K H Lim (SGH), S S Krisna (SGH), W T Lim NCCS), Y S Yap (NCCS), S C Lee (NUH), R A Soo (NUH), J Han (MIT/SMART), C T Lim (NUS/SMART), "Clinical Validation of an Ultra High-Throughput Spiral Microfluidics for the Detection and Enrichment of Viable Circulating Tumor Cells" PLoS One, PloS One, 9(7), e994092014. (2014)
- Yie Hou Lee (SMART), Chin Wen Tan (NUS/SMART), Abhishek Venkatratnaman (SMART), Chuen Seng Tan (NUH), Liang Cui (SMART), Seong Feei Loh (KKH), Linda Griffith (MIT/SMART), Steven R. Tannenbaum (MIT/SMART),
Jerry Kok Yen Chan (Duke-NUS/KKH), "Dysregulated sphingolipid metabolism in endometriosis", The Journal of Clinical Endocrinology and Metabolism, Jun 2014:jc20141340.
- Tze Ping Loh (NUH), Weng Kung Peng (SMART), Lan Chen (SMART), Sunil Kumar Sethi (NUH), "Application of smoothed continuous labile haemoglobin A1c reference intervals for identification of potentially spurious HbA1c results", Journal of Clinical Pathology, Published online on 11 June 2014 (doi:10.1136/jclinpath-2014-202346)
- Chin Wen Tan (NUS/SMART), Yie Hou Lee (SMART), Heng Hao Tan (KKH), Matthew Sie Kuei Lau (KKH), Mahesh Choolani (NUS), Linda Griffith (MIT/SMART), Jerry Kok Yen Chan (NUS/KKH/SMART/Duke-NUS), "CD26/DPPIV down-regulation in endometrial stromal cell migration in endometriosis", Published online in Fertility and Sterility (2014) (http://dx.doi.org/10.1016/j.fertnstert.2014.04.001)
- Majid Ebrahimi Warkiani (SMART), Bee Luan Khoo (MBI/NUS), Daniel Shao-Weng Tan (NCCS), Ali Asgar S. Bhagat (Clearbridge Biomedics), Wan-Teck Lim (NCCS), Yoon Sim Yap (NCCS), Soo Chin Lee (NUS), Ross A. Soo (NUS), Jongyoon Han (MIT/SMART), Chwee Teck Lim (NUS), "An ultra-high throughput spiral microfluidic biochip for the enrichment of circulating tumor cells", Analyst, 2014, Published online,
DOI: 10.1039/C4AN00355A
- Chee Ping Ng (SMART), Abdul Rahim Mohamed Sharif (SMART), Daniel E. Heath (SMART), John W. Chow,
Claire BY. Zhang, Mary B. Chan-Park (NTU), Paula T. Hammond (MIT/SMART), Jerry KY. Chan (Duke-NUS/KKH),
Linda G. Griffith (MIT/SMART), "Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM", Biomaterials, 35, 4046-4057 (2014)
- Toh Rou Jun (SMA3/NTU), Peng Weng Kung (SMART), Jongyoon Han (MIT/SMART), and Martin Pumera (NTU), “Haemoglobin electrochemical detection on various reduced graphene surfaces: well-defined glassy carbon electrode outperforms the graphenoids, RSC Advances, 4, 8050-8054 (2014)
- Zhou C (NTU), Heath DE (SMART), Sharif ARM (SMART), Rayatpisheh S (NTU), Oh B (NTU), Rong, X (NTU), Chan-Park, MBE (NTU), “High water content hydrogel with super high refractive index”, Macromolecular Bioscience, doi: 10.1002/mabi.201300191. [Epub ahead of print], July 2013
- Majid E. Warkiani, (SMART), Guofeng Guan (NUS), Khoo Bee Luan (NUS/MBI), Wong Cheng Lee (NUS), Ali Asgar S. Bhagat (CLearbridge Biomedics), Daniel Shao-Weng Tan (NCC), Soo Chin Lee, Wan Teck Lim (NCC/NUS), Peter C Y Chen (NUS), Chwee Teck Lim (NUS), Jongyoon Han (MIT/SMART). "Slanted spiral microfluidics for ultra-fast, label-free circulating tumor cells isolation", Lab on a chip, DOI: 10.1039/C3LC50617G, (Web), July 2013
- Ebrahimi Warkiani Majid (SMART) and Chwee Teck Lim (NUS), “Microfluidic Platforms for Human Disease Cell Mechanics Studies” – Book Chapter in Materiomics: Multiscale Mechanics of Biological Materials and Structures, CISM International Centre for Mechnical Sciences, Springer, Vol. 546, 2013,http://link.springer.com/book/10.1007/978-3-7091-1574-9/page/1
- Guofeng Guan (NUS/SMART) Lidan Wu (MIT), Ali Asgar Bhagat (SMART) Zirui Li (SMART), Petr C. Y. Chen, (NUS), Shuzhe Cao (NUS) Chng Jin Ong (NUS) andJonyoon Han (MIT/SMART), "Spiral microchannel with rectangular and trapezoidal cross-sections for size based particle separation", Scientific Reports (Nature Publishing), Published Online, March 2013
- Han Wei Hou (SMART/MIT), Majid Ebrahimi Warkiani (SMART), Bee Luan Khoo (MBI/NUS), Zi Rui Li (SMART), Ross A. Soo (CSIS, NUS/NUH),
Daniel Shao-Weng Tan (NCC), Wan-Teck Lim (NCC), Jongyoon Han (MIT/SMART), Ali Asgar S. Bhagat (SMART), and Chwee Teck Lim (NUS/MBI/SMART), "Isolation and retrieval of circulating tumor cells using centrifugal forces", Scientific Reports (Nature Publishing), Published Online, Feb 2013
- Kwak R (MIT), Guan G (NUS/SMART), Weng Kung Peng (SMART), Han J (MIT/SMART), “Microscale Electrodialysis: Concentration Profiling and Vortex Visualization,” Desalination, 308, 138 (2013)
- Paradise, RK (MIT/SMART), Whitfield, M (MIT/SMART), Lauffenburger, DA (MIT), and Van Vliet, KJ (MIT/SMART), “Directional cell migration in an extracellular pH gradient: A model study with an engineered cell line and primary microvascular endothelial cells,” Experimental Cell Research, Nov 12. pii: S0014-4827(12)00452-1. doi: 10.1016/j.yexcr.2012.11.006. [Epub ahead of print]
- Guofeng Guan (NUS/SMART), Chao Yu Peter Chen (NUS), Weng Kung Peng (SMART), Ali Asgar Bhagat (SMART), Chong Jin Ong (NUS), Jongyoon Han (MIT/SMART), “Real-time control of a microfluidic channel for size-independent deformability cytometry,” Journal of Micromechanics and Microengineering, 22, 105037 (2012).
- Weng Kung Peng (SMART), Chen Lan (SMART), and Jongyoon Han (MIT/SMART), “Development of miniaturized, portable Magnetic Resonance Relaxometry System for Point-of-Care Medical Diagnosis,” Review of Scientific Instruments, 83, 095115 (2012)
- Li L (MIT), Lieleg O (MIT), Jang S (MIT), Ribbeck K (MIT), Han J (MIT/SMART), “A microfluidic in vitro system for quantitative study of stomach mucus barrier function,” Lab on a Chip, 12, 4071 (2012).
- Diez-Silva M, Park Y, Huang S, Bow H, Mercereau-Puijalon O, Deplaine G, Lavazec C, Perrot S, Bonnefoy S, Feld MS, Han J (SMART/MIT), Dao M, Suresh S. “Pf155/RESA protein influences the dynamic microcirculatory behavior of ring-stage Plasmodium falciparum infected red blood cells,” Scientific Reports, 2 : 614 | DOI: 10.1038/srep00614 (2012).
- Han Wei Hou (NUS/SMART), Hiong Yap Gan (MIT), Ali Asgar S. Bhagat (SMART), Leon D. Li (MIT), Chwee Teck Lim (NUS), and Jongyoon Han (MIT/SMART), "A Microfluidics Approach Towards High-Throughput Pathogen Removal From Blood", Biomicrofluidics, 6, 024115, 2012. (DOI: 10.1063/1.4710992) (also selected for the May 14, 2012 issue of Virtual Journal of Nanoscale Science & Technology)
- Tian Fook Kong (NTU/SMART), Weng Kung Peng (SMART), Trung Dung Luong (NTU), Nam-Trung Nguyen (NTU) and Jongyoon Han (MIT/SMART), "Adhesive-based liquid metal radio-frequency microcoil for magnetic resonance relaxometry measurement", Lab on a Chip,12, 287-294 (2012)
- H.W. Hou (NUS/SMART), A.A.S. Bhagat (SMART), W.C. Lee (NUS), S. Huang (MIT), J. Han (MIT/SMART) and C. T. Lim (NUS), "Microfluidic devices for blood fractionation", Micromachines, 2011. 2(3): p. 319-343.
- A.A.S. Bhagat (SMART), H.W. Hou (SMART/NUS), Leon. D. Li (MIT), C.T. Lim (NUS) and J. Han (SMART/MIT) "Pinched flow coupled shear-modulated inertial microfluidics for high-throughput rare blood cell separation", Lab on a Chip, 2011 (DOI: 10.1039/c01c00633e).
- Wong Cheng Lee (NUS/SMART), Ali Asgar. S. Bhagat (SMART), Huang Sha (MIT), Krystyn J. Van Vliet (MIT/SMART), Jongyoon Han (MIT/SMART), Chwee Teck Lim (NUS), "High-throughput cell cycle synchronization using inertial forces in spiral microchannels," Lab on a Chip, 11, 1359 (2011)
- H.Bow, I.Pivkin, M.Diez-Silva, S.J.Goldfless, M.Dao, J.C.Niles, S.Suresh, and J.Han "A microfabricated deformability-based flow cytometer with application to malaria", Lab on a Chip 11: 1065 (2011).
- R.K.Paradise, D.A.Lauffenburger, and K.J.Van Vliet, “Acidic extracellular pH promotes activation of integrin avb3,” PLoS ONE 6 e15746(2011)
- H.W. Hou, A.A.S. Bhagat, A.G.L. Chong, P. Mao P, K.S.W. Tan, J. Han and C.T. Lim, "Deformability based cell margination – A simple microfluidic design for high throughput malaria infected erythrocyte separation", Lab on a Chip, vol. 10, 2605 (2010).
- Ali Asgar S. Bhagat, Hansen Bow, Han Wei Hou, Swee Jin Tan, Jongyoon Han, Chwee Teck Lim, "Microfluidics for cell separation", - SPECIAL ISSUE - REVIEW, Med Biol Eng Comput, 48, 999 (2010).
|
|
- Khoo Bee Luan (NUS/MBI), Majid Ebrahimi Warkiani (SMART), Jongyoon Han (MIT), Chwee Teck Lim (NUS), "Multiplexed spiral inertial microfluidics for the high-throughput and label-free enrichment of viable circulating tumor cells", 7th WACBE World Congress on BioEngineering 2015, Singapore, 6-8 July 2015
- W. Peng (SMART), C. Ng (NUH), T. Kong (NTU/SMART), L. Chen (SMART), T. Loh (NUH), P. Preiser (NTU), and J. Han (MIT/SMART), "Micro Magnetic Resonance Relaxometry for Label-free, Rapid Malaria Diagnosis, Biomedical Engineering Society, 2014 Annual Meeting, San Antonio, USA, Oct, 2014
- M. Ebrahimi Warkiani (SMART), A.K.P. Tay (NUS), B.L. Khoo (NUS/MBI), X. Xiaofeng (NUS), C.T. Lim (NUS/SMART), and J. Han (MIT/SMART), “ Enabling reliable detection of low abundance malaria parasites from blood using inertial microfluidics”, MicroTAS-2014, San Antonio, USA, Oct, 2014.
- M. Ebrahimi Warkiani (SMART), A.K.P. Tay (NUS), Guan Guofeng (NUS), and J. Han (MIT/SMART), “Next generation microfilter: Large scale, continuous mammalian cells for perfusion bio-reactors”, MicroTAS-2014, San Antonio, USA, Oct, 2014.
- Weng Kung Peng (SMART), Jongyoon Han (MIT/SMART), "Development of novel marker and portable magnetic resonance relaxometry system for Point of Care Medical diagnosis", Proceedings of the 6th International Symposium on Microchemistry and Microsystems (ISMM), pp 104-105, 30 July – 1 August 2014,
Singapore
- W. Lee (SMART), H. Shi (SMART), Z. Poon (SMART), J. Chan (Duke-NUS/KKH), G.
Shivashankar (MBI/NUS), C. Lim (NUS), J. Han (MIT/SMART), K. Van Vliet (MIT/SMART), "Stem Cell Heterogeneity: Identifying Subsets of Mesenchymal Stromal Cells for Tissue Engineering & Translational Medicine", 7th World Congress of Biomechanics, July 6-11, 2014, Boston, MA
- Z. Poon (SMART), J. Lee W.C. (SMART), K. Van Vliet (MIT/SMART), "Biophysically derived osteoprogenitors from mesenchymal stem/stromal cell (MSC) cultures for applications in orthopedics and hematology", 7th World Congress of Biomechanics, July 6-11, 2014, Boston, MA
- Yie Hou Lee (SMART), Chin Wen Tan (NUS/SMART), Linda Griffith (MIT), Steven R. Tannenbaum (MIT/SMART), Jerry Kok Yen Chan (Duke-NUS/KKH), "Maternal peritoneal fluid sphingolipids in endometriosis-associated infertility", World Congress on Endometriosis 2014, Sao Paulo, Brazil
30 April - 3 May 2014
- D.E. Heath (SMART), C.P. Ng (SMART), Sharif ARM (SMART), M.B.E. Chan-Park (NTU), P.T. Hammond (MIT/SMART), L.G. Griffith (MIT/SMART), “High Aspect Ratio Hydrogel Microwell Arrays As a Novel Platform for the In Situ Cloning of Rare Cells”, American Institute of Chemical Engineering Annual Meeting, San Francisco, CA, Nov- 2013
- D.E. Heath (SMART), C.P. Ng (SMART), A.R.M. Sharif (SMART), M.B.E. Chan-Park (NTU), P.T. Hammond (MIT/SMART), L.G. Griffith (MIT/SMART), “Hydrogel microwell arrays for in situ culture and analysis of single cells”, Biomedical Engineering Society Annual Meeting, Seattle, WA, USA Sept-2013
- T. Jing (NUS/SMART), R. Ramji (NUS), M.E. Warkiani (SMART), C.T. Lim (NUS), J. Han (MIT/SMART), and C.-H. Chen (NUS/SiNAPSE), High throughput single cancer cell encapsulation and self sorting for protease assy by using jetting microfluidics, MicroTAS 2013, Germany
- Tian Fook Kong (SMA3/NTU), Weng Kung Peng (SMART), Han Wei Hou (MIT), Marcos (NTU), Nam-Trung Nguyen (Grififth), and Jongyoon Han (MIT/SMART), "Rapid and high sensitivity malaria diagnosis: a microfluidics approach", MicroTAS 2013, Germany.
- Guofeng Guan (NUS), Majid E. Warkiani, (SMART), Khoo Bee Luan (NUS/MBI), Daniel Shao-Weng Tan (NCC), Ali Asgar S. Bhagat (Clearbridge Biomedics), Wan-Teck Lim (NCC/NUS), Peter C. Y. Chen (NUS), Jongyoon Han (MIT/SMART), Chwee Teck Lim (NUS), "High throughput circulating tumor cell isolation using trapezoidal inertial microfluidics", MicroTAS 2013, Germany.
- Majid Ebrahimi Warkiani (SMART), Khoo Bee Luan (NUS/MBI), Daniel Shao-Weng Tan (NCC), Ali Asgar S. Bhagat (Clearbridge Biomedics), Wan-Teck Lim (NCC/NUS), Jongyoon Han (MIT/SMART), Chwee Teck Lim (NUS), "Circulating Tumor Cell (CTC) enrichment: Ultra high throughput processing of clinically relevant blood volumes using a multiplexed spiral biochip", MicroTAS 2013, Germany.
- C. Kantak (NUS), S. Beyer (SMART), and D. Trau (NUS), "A novel microfluidic droplet manipulation method for fabrication of reverse-phase two layer-by-layer protein microcapsules", MicroTAS 2013, Germany.
- R. Kwak (MIT), V.S. Pham (MIT), B.J. Kim (MIT),
L. Chen (SMART), and J. Han (MIT/SMART), "High throughput salt/bio-agent removal by ion concentration polarization for water desalination, purification and monitoring, MicroTAS 2013, Germany.
- Y.H. Lee (SMART), S.F. Loh (KKH), S.R. Tannenbaum (MIT/SMART), J.K.Y. Chan (Duke-NUS/KKH), “Dysregulated sphingolipid metabolism promotes cell growth in endometriosis”, 29th Annual Meeting: European Society of Human Reproduction and Embryology, London, UK, July 2013
- C.W. Tan (SMA3/NUS), Y.H. Lee (SMART), H.H. Tan (KKH), M. Choolani (NUS), Linda. Griffith (MIT/SMART), J.K.Y. Chan (Duke-NUS/KKH), “Decidualisation of fetal bone marrow derived mesenchymal stem cells is gender dependent”, International Society of Stem Cell Society, Annual Meeting, Boston, USA, June 2013
- Krystyn Van Vliet (MIT/SMART), “Biological Systems & Micromechanics: New tools to identify key cells and molecules,” IBN is 10 Symposium, January 2013, A*STAR Institute for Bio and Nano, Biopolis, Singapore.
- Hui Shi (SMART), Wong Cheng Lee (NUS), Zhiyong Poon (SMART), G. Shivashankar (MBI/NUS), Chwee Teck Lim (NUS), Jongyoon Han (MIT/SMART) and Krystyn J. Van Vliet (MIT/SMART), “Correlation of single-cell biophysical properties with cell function”, IBN is 10 Symposium, January 2013, A*STAR Institute for Bio and Nano, Biopolis, Singapore.
- Daniel E. Heath (SMART), Chee Ping Ng (SMART), Sharif Mohamed (SMART), Xinyan Liu (NUS), Paula T. Hammond (MIT/SMART), Linda G. Griffith (MIT/SMART), Mary B. Chan (NTU), "Hydrogel microwell arrays for the isolation, culture, and analysis of rare cell populations", Biomedical Engineering Society (BMES) Meeting, Atlanta, GA, Oct-2012
- Chee Ping Ng (SMART), Daniel E. Heath (SMART), Sharif Mohamed (SMART), Xinyan Liu (NUS), Mary B. Chan (NTU), Paula T. Hammond (SMART/MIT), Linda G. Griffith (MIT/SMART), "Novel microwell colony forming unit (CFU) assays for bone marrow stromal cells", Biomedical Engineering Society (BMES) Meeting, Atlanta, GA, Oct-2012
- Guofeng Guan (NUS), Ali Asgar Bhagat (SMART), Lidan Wu (MIT), Zirui Li (SMART), Chong Jin Ong (NUS), Peter C. Y. Chen (NUS), Jongyoon Han (MIT/SMART), "High resolution size based microparticle / cell separator with trepezoid cross section spiral microchannels", MicroTAS-2012, Oct., 2012
- Lidan Wu (MIT), Guofeng Guan (NUS), Hanwei Hou (SMART), Ali Asgar. S. Bhagat (SMART), Jongyoon Han (MIT/SMART),"Continuous neutrophil isolation from blood using spiral channel with trepezoid cross-section", MicroTAS-2012, Oct., 2012
- Majid Ebrahimi Warkiani (SMART), Bee Luan Khoo (NUS), Ali Asgar. S. Bhagat (SMART), Jongyoon Han (MIT/SMART), Chwee Teck Lim (NUS), "Ultra high-throughput separation of circulating tumor cells (CTCs) from blood using a novel microfluidic device", Advances in Circulating Tumour Cells (ACTC), Sept-2012, Athens, Greece
- W. K. Peng (SMART), and J. Han (MIT/SMART), "Micro Magnetic Resonance Relaxometry for Label-free Rapid Malaria Diagnosis" Annual Meeting of Biomedical Engineering Society (BMES) @ Connecticut, USA, Oct 2011.
- A.A.S. Bhagat (SMART), H.W. Hou (NUS/SMART), L.D. Li (MIT), C.T. Lim (NUS), and J. Han (MIT/SMART), “Dean flow fractionation (DFF) for isolating circulating tumor cells (CTCs) from high-hematocrit blood samples”, MicroTAS 2011, Seattle, USA, October 2-6, 2011, pp. 524-526.
- Han Wei Hou (NUS/SMART), H.Y.Gan (MIT), Ali Asgar Bhagat (SMART), L.D.Li (MIT), Chwee Teck Lim (NUS) and Jongyoon Han (MIT/SMART), "Removal of pathogen and inflammatory components from blood using cell margination, MicroTAS 2011, Seattle, USA, October 2-6, 2011, pp. 1912-1914.
- G.Guan (NUS), A.B. Bhagat (SMART), W.K. Peng (SMART), W.C. Lee (NUS), C.J. Ong (NUS), C.Y. Chen (NUS), and J. Han (MIT/SMART), "Size-Independent Deformability Cytometer with Active Feedback Control of Microfluidic Channels” MicroTAS 2011, Seattle, USA, October 2-6, 2011, pp. 1053-1055
- Leon Li (MIT), Oliver Lieleg (MIT), Katharina Ribbeck (MIT), Jongyoon Han(MIT/SMART), Microfluidic “Microfluidic In VitroModel for Quantitative Study of Stomach Mucin Acid Barrier Function” MicroTAS 2011, Seattle, USA, October 2-6, 2011, pp. 881-773.
- P.W. Kung (SMART), A.A.S.Bhagat (SMART), J.Han (MIT/SMART), "µMagnetic Resonance Relaxometry for label-free, rapid malaria diagnosis", 52nd Experimental Nuclear Magnetic Resonance Conference, April 2011, Asilomar.
- Wong Cheng Lee, Ali Asgar S. Bhagat, Sha Huang, Krystyn Van Vliet, Jongyoon Han and Chwee Teck Lim, “High throughput selection of stem cells into different phases of the cell cycle using inertial microfluidics,” in 2nd Conference on Advances in Microfluidics and Nanofluidics and Asian Pacific International Symposium on Lab on chip (AMN-APLOC 2011), Singapore, January 5-7, 2011.
- Han Wei Hou, Ali Asgar. S.Bhagat, Pan Mao, Jongyoon Han and Chwee Teck Lim, "Deformability based cell margination for malarial infected red blood cell enrichment, MicroTAS-2010, Groningen, Netherlands, Oct-2010
- Wong Cheng Lee, Ali Asgar. S. Bhagat, Sha Huang, Krystyn Van Vliet, Jongyoon Han and Chwee Teck Lim, "Cell cycle synchronization of stem cells using inertial microfluidics" MicroTAS-2010, Groningen, Netherlands, Oct-2010
- Han Wei Hou, Ali Asgar. S. Bhagat, Pan Mao, Chwee Teck Lim, Jongyoon Han, "High-throughput circulating tumor cells (CTCs) isolation using inertial forces" MicroTAS-2010, Groningen, Netherlands, Oct-2010
- Sha Huang (MIT/SMART), Hansen Bow (MIT/SMART), Monica Diez-Silva (MIT), and Jongyoon Han (MIT/SMART), “Applying Microfluidic ‘Deformability Cytometry’ to measure stiffness of malaria-infected red blood cells at body and febrile temperatures”, MicroTAS-2010, Groningen, Netherlands, Oct-2010
- Han Wei Hou, Ali Asgar Bhagat, Jongyoon Han and Chwee Teck Lim , "Deformability based cell margination - A simple microfluidic design for malarial infected red blood cell filtration", 6th World Congress of Biomechanics, 1-6 August 2010, Singapore, Proceedings pp.556
- Ali Asgar Bhagat, Han Wei Hou, Chwee Teck Limand Jongyoon Han, "Inertial based spiral spiral microfluidics for isolating circulating tumor cells (CTCs) from blood", 6th World Congress of Biomechanics, 1-6 August 2010, Singapore, Proceedings pp.556
- Wong Chee Lee, Ali Asgar Bhagat, Jongyoon Han and Chwee Teck Lim, "Inertial based spiral microfluidics for fractionating stem cells into different stages of cell cycles", 6th World Congress of Biomechanics, 1-6 August 2010, Singapore, Proceedings pp.561
- Sha Huang, Hansen Bow, Monica Diez-Silva , and Jongyoon Han , “Study of Temperature-Dependent Deformability of Red Blood Cells Using Microfluidic Bottleneck Array”, 6th World Congress of Biomechanics, 1-6 August 2010, Singapore
- H.W. Hou, A. A. S. Bhagat, J. Han and C. T. Lim, "Deformability based cell margination - A simple microfluidic design for malarial infected red blood cell filtration", 25th International Symposium on Microscale Bioseparations (MSB2010), Prague, March 21-25, 2010.
- Bow, H., H.W. Hou, S. Goldfless, P. Abgrall, K. Tan, J. Niles, C.T. Lim, and J. Han, "Continuous Flow Deformability Based Sorting of Malaria Infected Red Blood Cells", MicroTAS-2009, Jeju, Korea. pp. 1219-1221
|
|
 |
















 A
A B
B

